Group
 Webster Group
Webster GroupDepartment of Chemistry
Mississippi State University
Phone: (662) 325-7224
ewebster@chemistry.msstate.edu
Example 1: β-Phosphoglucomutase
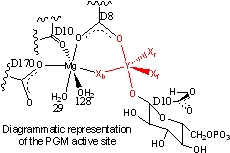
Lahiri et al. reported the crystal structure of β-phosphoglucomutase (PGM); it was refined as a five-coordinate phosphorus (with five oxygen ligands, which the authors suggested was a "high-energy reaction intermediate" for phosphoryl transfer in the isomerization of β-glucose 1-phosphate to β-glucose 6-phosphate. The active site of PGM was reported to contain a five-coordinate atom (Y=P) and a pseudo-octahedral Mg, coordinated by four terminal ligands (two waters, one carboxylate oxygen from ASP170, and one backbone carboxyate oxygen from ASP10) and two bridging ligands (one of the carboxylate oxygens from ASP8 and an atom, Xb, bridging to the five-coordinate species, Y, see Scheme). Blackburn et al. later suggested that this structure was actually a transition-state analogue with a five-coordinate magnesium (Y=Mg) with two oxygen and three fluorine ligands.
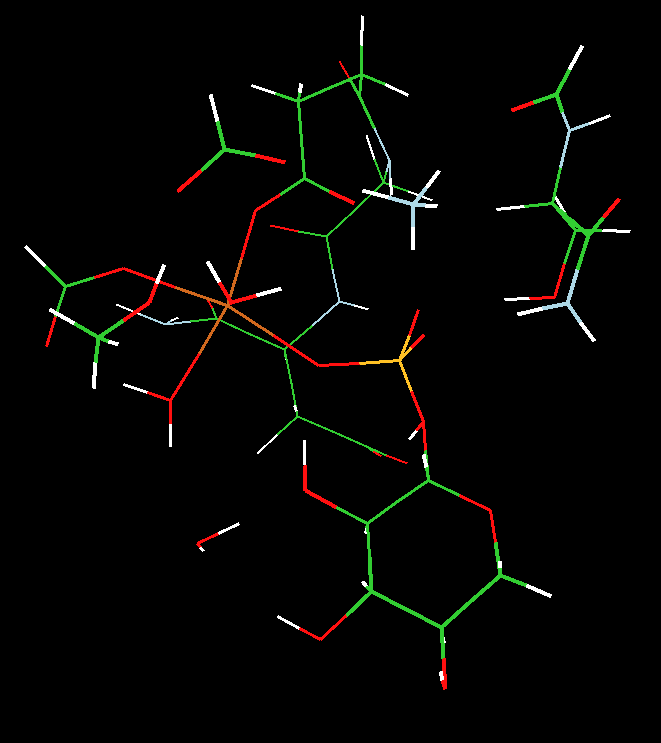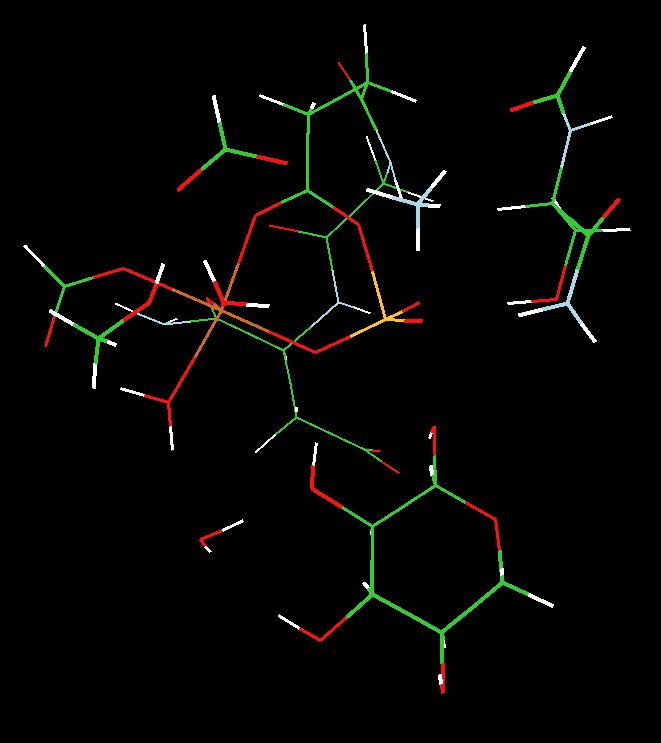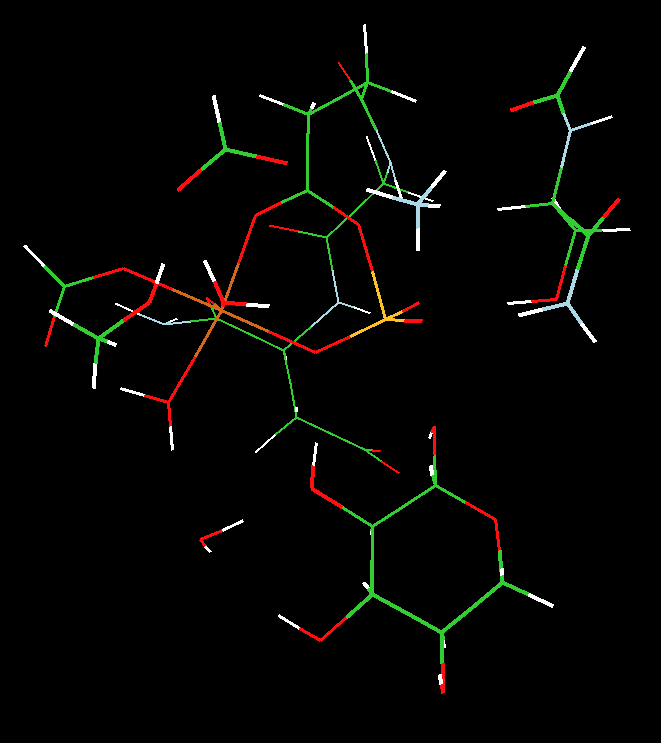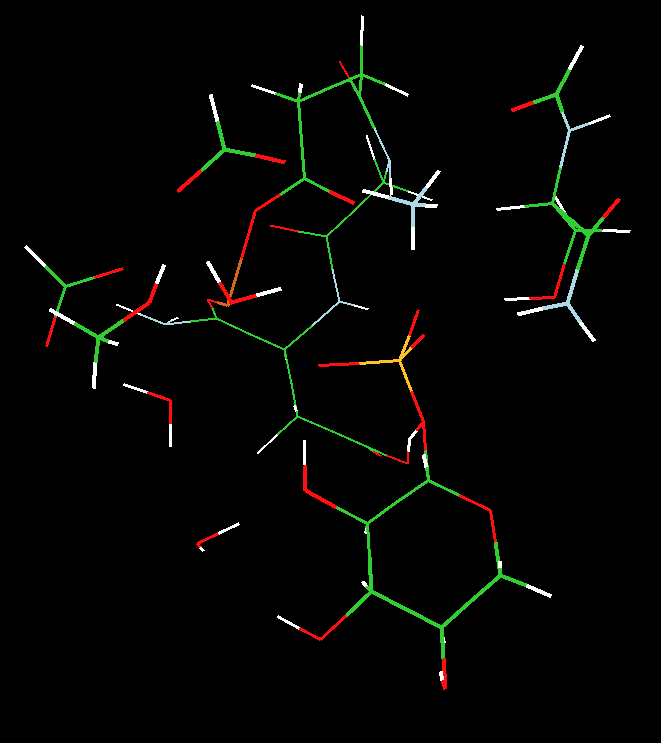
We utilized two-layered ONIOM(MO:MO) B3LYP density functional theory and semi-empirical PM3 calculations to address the nature of the PGM enzyme active site structure and shed light on the identity of the five coordinate atom, Y, and its ligation (see Scheme).
We concluded that 1) the observed crystal structure was more consistent with a five-coordinate magnesium (a stable transition-state analogue), not a five-coordinate phosphorus (a phosphorane) and 2) the transfer of the phosphoryl group proceeds through a concerted five-coordinate phosphorus transition state that is directly coupled to a proton transfer from the oxygen of bound glucose to the carboxylic group of aspartate 10.
The animation is the imaginary mode that corresponds to the transition state for phosphoryl tranfer with concomitant proton transfer from the hydroxyl sugar to ASP10 oxygen.
- Lahiri, Zhang, Dunaway-Mariano, and Allen Science 2003, 299, 2067-2071.
- Allen and Dunaway-Mariano Science 2003, 301, 1184d.
- Blackburn, Williams, Gamblin, and Smerdon Science 2003, 301, 1184c.
- Webster J. Am. Chem. Soc. 2004, 126, 6840-6841.