Group
 Webster Group
Webster GroupDepartment of Chemistry
Mississippi State University
Phone: (662) 325-7224
ewebster@chemistry.msstate.edu
Example 2: Photochromic Organometallics
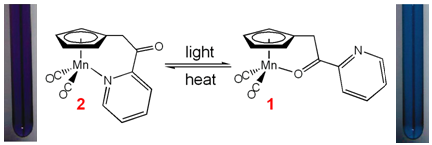 In collaboration with Ted Burkey's group at the Univeristy of Memphis, we have the studied the linkage isomerization
mechanism for the photochromic derivatives of (C5H4R)Mn(CO)3 with chelatable R-functional groups.
UV irradiation of the tricarbonyl complex causes CO dissociation. Both pyridyl (2) and carbonyl (1)
chelates are observed following irradiation.
In collaboration with Ted Burkey's group at the Univeristy of Memphis, we have the studied the linkage isomerization
mechanism for the photochromic derivatives of (C5H4R)Mn(CO)3 with chelatable R-functional groups.
UV irradiation of the tricarbonyl complex causes CO dissociation. Both pyridyl (2) and carbonyl (1)
chelates are observed following irradiation.
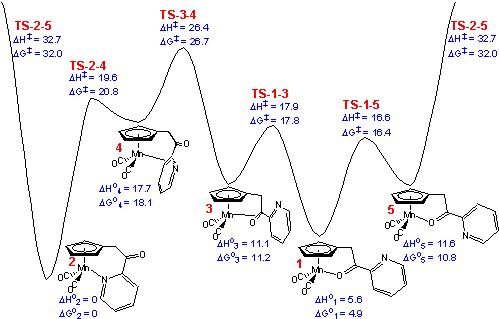 Based upon the results from PBE density functional theory calculations,
the lowest-energy isomerization pathway from 1 (the carbonyl chelate) to 2
(the pyridine chelate) proceeds via three steps: 1 (the lower-energy
oxygen-bound linkage isomer with a carbonyl to pyridine σ-trans
conformation) to 3 (an η2 π-bound
carbonyl intermediate), 3 to 4 (an η2 π
-bound pyridyl intermediate), and 4 to 2
(the nitrogen-bound linkage isomer). The computed activation enthalpy is 20.8
kcal/mol (1 to TS-3-4). Confirmation of the calculations were obtained from kinetic
experiments where the rates of decay of proton NMR peaks for 1 and
recovery of those for 2 were found to be equal and first order in 1.
The corresponding experimental activation enthalpies obtained from Eyring plots
were in good agreement with the computed activation enthalpy (22.0 ± 0.5
and 20.8 ± 0.2 kcal/mol, respectively). Furthermore, the computational ΔH‡
and ΔG‡ are quite similar in value indicating that TΔS‡ is
small (-1.0 kcal/mol at 25 °C). This value is also in good agreement with the
small experimental TΔS‡ (1.0 kcal/mol) which was obtained from the
experimental activation entropy of 3.5 ± 0.1 eu (calculated using the average experimental activation enthalpy of
21.4 ± 0.8 kcal/mol). A low activation entropy is consistent with a pathway where the side
chain never dissociates from the metal as proposed for 1→3→4→2.
The computational results for intermediates 3 with an η2
metal-carbonyl interaction and likewise 4 with an η2 metal-pyridine interaction that does not involve
the nitrogen suggest a mechanism where the metal walks along the π bonds of
the side chain from one functional group to another instead of complete dissociation
from the side chain followed by addition of the pyridyl group. Computational
results indicate that dissociation of the side-chain functional group to form a
coordinatively-unsaturated 16-electron complex followed by rotation and
coordination by the second functional group is higher in energy than the π-bound
pathway. A two-step pathway from 1 to 2 was also found: from 1
to 5 (the higher-energy cis conformer of the oxygen-bound
linkage isomer) and from 5 to 2. However, this two-step pathway (1→5→2) is higher in energy by more
than 6 kcal/mol compared to the π-bound pathway.
Based upon the results from PBE density functional theory calculations,
the lowest-energy isomerization pathway from 1 (the carbonyl chelate) to 2
(the pyridine chelate) proceeds via three steps: 1 (the lower-energy
oxygen-bound linkage isomer with a carbonyl to pyridine σ-trans
conformation) to 3 (an η2 π-bound
carbonyl intermediate), 3 to 4 (an η2 π
-bound pyridyl intermediate), and 4 to 2
(the nitrogen-bound linkage isomer). The computed activation enthalpy is 20.8
kcal/mol (1 to TS-3-4). Confirmation of the calculations were obtained from kinetic
experiments where the rates of decay of proton NMR peaks for 1 and
recovery of those for 2 were found to be equal and first order in 1.
The corresponding experimental activation enthalpies obtained from Eyring plots
were in good agreement with the computed activation enthalpy (22.0 ± 0.5
and 20.8 ± 0.2 kcal/mol, respectively). Furthermore, the computational ΔH‡
and ΔG‡ are quite similar in value indicating that TΔS‡ is
small (-1.0 kcal/mol at 25 °C). This value is also in good agreement with the
small experimental TΔS‡ (1.0 kcal/mol) which was obtained from the
experimental activation entropy of 3.5 ± 0.1 eu (calculated using the average experimental activation enthalpy of
21.4 ± 0.8 kcal/mol). A low activation entropy is consistent with a pathway where the side
chain never dissociates from the metal as proposed for 1→3→4→2.
The computational results for intermediates 3 with an η2
metal-carbonyl interaction and likewise 4 with an η2 metal-pyridine interaction that does not involve
the nitrogen suggest a mechanism where the metal walks along the π bonds of
the side chain from one functional group to another instead of complete dissociation
from the side chain followed by addition of the pyridyl group. Computational
results indicate that dissociation of the side-chain functional group to form a
coordinatively-unsaturated 16-electron complex followed by rotation and
coordination by the second functional group is higher in energy than the π-bound
pathway. A two-step pathway from 1 to 2 was also found: from 1
to 5 (the higher-energy cis conformer of the oxygen-bound
linkage isomer) and from 5 to 2. However, this two-step pathway (1→5→2) is higher in energy by more
than 6 kcal/mol compared to the π-bound pathway.
- To, Duke, Junker, O'Brien, Ross, Barnes, Webster, Burkey Organometallics, 2008, 27, 289-296.