Group
 Webster Group
Webster GroupDepartment of Chemistry
Mississippi State University
Phone: (662) 325-7224
ewebster@chemistry.msstate.edu
Example 3: Ethylene Exchange in Grubb’s Catalysts
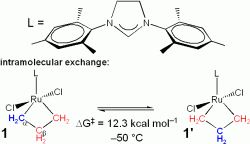
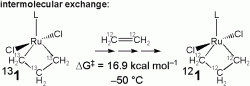 Olefin metathesis reactions, which exchange the substituents
about the double bonds of the alkenes, are important for a variety of chemical
syntheses. A metallocyclobutane intermediate has been implicated in the
mechanism of the Grubbs-type catalyzed olefin metathesis. Romero and Piers
reported an experimental mechanistic study of ethylene exchange with a
14-electron ruthenacyclobutane, (NHC)Cl2Ru(CH2CH2CH2),
where NHC is an N-heterocyclic carbene (1,3-dimesitylimidazolidin-2-ylidene),
derived from a second generation Grubbs catalyst, see Scheme. They described
1) intramolecular exchange of Cα
and Cβ in 1 and 2)
intermolecular exchange, the degenerate exchange of free ethylene with 1.
Olefin metathesis reactions, which exchange the substituents
about the double bonds of the alkenes, are important for a variety of chemical
syntheses. A metallocyclobutane intermediate has been implicated in the
mechanism of the Grubbs-type catalyzed olefin metathesis. Romero and Piers
reported an experimental mechanistic study of ethylene exchange with a
14-electron ruthenacyclobutane, (NHC)Cl2Ru(CH2CH2CH2),
where NHC is an N-heterocyclic carbene (1,3-dimesitylimidazolidin-2-ylidene),
derived from a second generation Grubbs catalyst, see Scheme. They described
1) intramolecular exchange of Cα
and Cβ in 1 and 2)
intermolecular exchange, the degenerate exchange of free ethylene with 1.
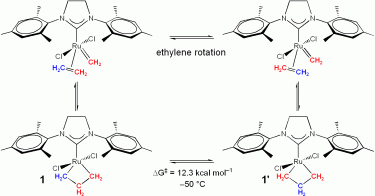 For intramolecular carbon exchange, one could envision a three-step mechanism that
would in the first step cleave the metallocyclobutane C-C bond (to form a methylene, ethylene complex),
in the second step rotate the coordinated ethylene, and in the third step form the C-C bond (to
form the metallocyclobutane, with exchanged α
and β carbons). This three-step proposed
mechanism is quite reasonable.
For intramolecular carbon exchange, one could envision a three-step mechanism that
would in the first step cleave the metallocyclobutane C-C bond (to form a methylene, ethylene complex),
in the second step rotate the coordinated ethylene, and in the third step form the C-C bond (to
form the metallocyclobutane, with exchanged α
and β carbons). This three-step proposed
mechanism is quite reasonable.
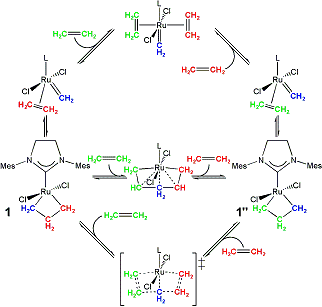 For intermolecular ethylene exchange, one could envision a
variety of mechanisms. Ethylene could bind to 1 or 2 and a
postulated metallocylohexane transition state or intermediate could be involved
in the exchange. These structures could have either trans-disposed or
cis-disposed chloride analogues (our computational results indicate a multistep
mechanism with structures that have trans-disposed, not cis-disposed chlorides).
For intermolecular ethylene exchange, one could envision a
variety of mechanisms. Ethylene could bind to 1 or 2 and a
postulated metallocylohexane transition state or intermediate could be involved
in the exchange. These structures could have either trans-disposed or
cis-disposed chloride analogues (our computational results indicate a multistep
mechanism with structures that have trans-disposed, not cis-disposed chlorides).
We applied PBE density functional theory calculations to test various mechanisms and consider dissociative versus associative mechanisms at elevated temperatures at which these olefin metathesis catalysts are also used.
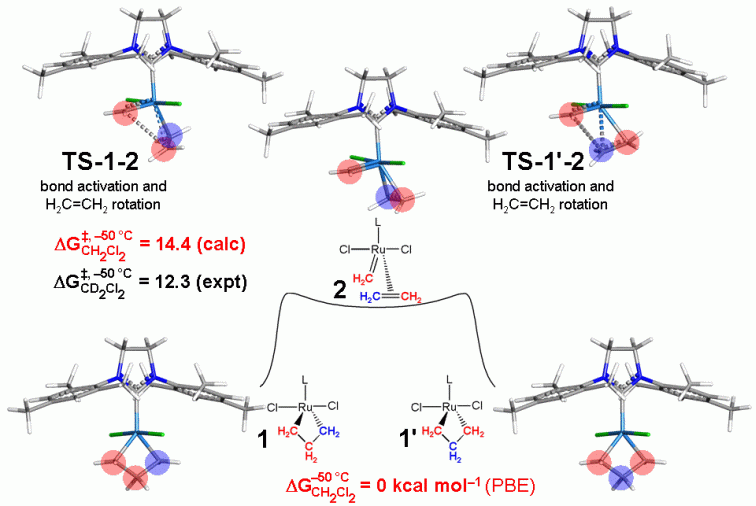
For intramolecular ethylene exchange, our computational results indicate a single-step mechanism (to form a methylene, ethylene complex) starting from the ruthenacyclobutane complex (1) directly producing the ethylene ruthenium carbene complex (2) by a rotational bond-breaking transition state (TS-1-2, see Figure directly above).
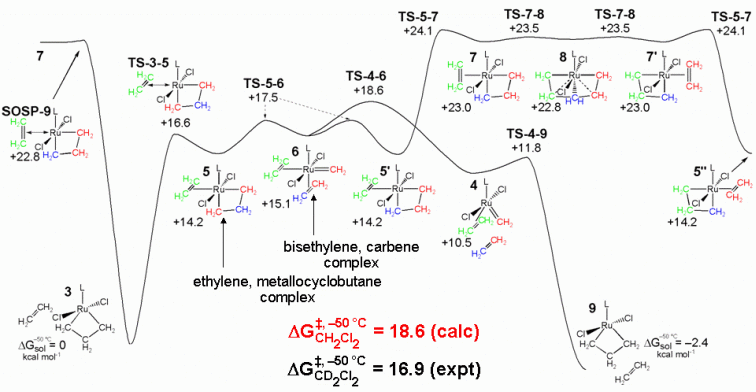
For intermolecular ethylene exchange, our computational results indicate a multi-step mechanism starting from 3 (ethylene associated with 1), ethylene binds to the metal (through TS-3-5), which produces the η2-ethylene ruthenacyclobutane complex (5). A rotational bond-breaking transition state (TS-5-6) produces 6 (a bis ethylene methylene complex), from which the ethylene trans to NHC can dissociate (through TS-4-6). A second ruthenacyclobutane complex with associated ethylene (9) is formed through TS-4-9 (similar to TS-1-2). While the proposed pathway is not an energetically symmetric pathway, it does not violate the principle of microscopic reversibility.
An alternative mechanism which involves a ruthenacyclohexane intermediate proceeds from 5, η2-ethylene rotates (through TS-5-7) to form 7. A high-energy ruthenacyclohexane intermediate (8) is formed through TS-7-8. This pathway with a ruthenacyclohexane intermediate is higher in energy than the first described intermolecular ethylene exchange pathway.
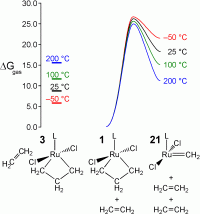 Romero and Piers suggested that a competing mechanism
involving dissociation of ethylene from 1 at elevated temperatures could
become competitive with an associative mechanism. To test that suggestion, the
relative free energy of ethylene loss from 1 was computed. A rise of the
temperature to 100 °C (from -50 °C) increases the stability of separated
ethylene and the methylene fragment by ~11.8 kcal mol-1 when
compared to 3. Therefore, ethylene dissociation from 1 could
become the operative pathway at elevated temperatures.
Romero and Piers suggested that a competing mechanism
involving dissociation of ethylene from 1 at elevated temperatures could
become competitive with an associative mechanism. To test that suggestion, the
relative free energy of ethylene loss from 1 was computed. A rise of the
temperature to 100 °C (from -50 °C) increases the stability of separated
ethylene and the methylene fragment by ~11.8 kcal mol-1 when
compared to 3. Therefore, ethylene dissociation from 1 could
become the operative pathway at elevated temperatures.
- Webster, J. Am. Chem. Soc. 2007, 129, 7490-7491.
- Grubbs, Ed. Handbook of Metathesis; Wiley-VCH: New York, 2003.
- Trnka, Grubbs, Acc. Chem. Res. 2001, 34, 18-29.
- Schrock, Hoveyda, Angew. Chem. Int. Ed. 2003, 42, 4592-4633.
- Schrock, Angew. Chem., Int. Ed. 2006, 45, 3748-3759.
- Grubbs, Angew. Chem., Int. Ed. 2006, 45, 3760-3765.
- Romero, Piers, J. Am. Chem. Soc. 2007, 129, 1698-1704.
- Anderson, Hickstein, O'Leary, Grubbs, J. Am. Chem. Soc. 2006, 128, 8386-8387.
- Romero, Piers, J. Am. Chem. Soc. 2005, 127, 5032-5033.